Singapore approves interim use of 1st bivalent COVID-19 booster vaccine
Sep 15, 2022
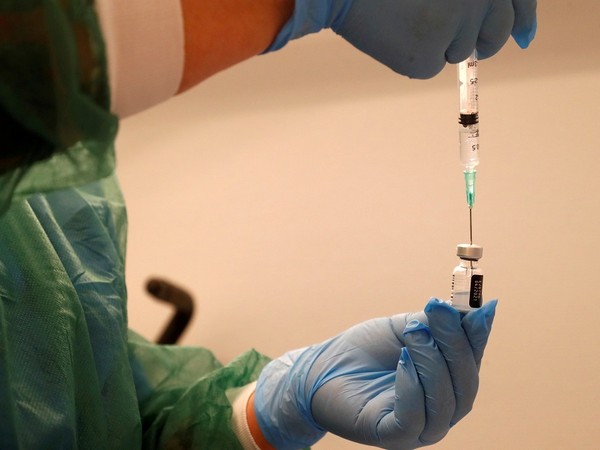
Singapore, September 15: Singapore's Health Sciences Authority (HSA) has granted interim authorization for use of a bivalent COVID-19 booster vaccine in Singapore, the first of its kind approved in the country.
The Spikevax Bivalent Original/Omicron COVID-19 vaccine produced by Moderna comprises two components that target the original SARS-CoV-2 strain and the Omicron BA.1 variant respectively, the HSA said in an announcement published on its website on Wednesday.
It is authorized for use as a booster vaccine in individuals aged 18 years and above, who have received primary series vaccination with COVID-19 vaccines, said the HSA.
The HSA said that it had carefully reviewed the data from Moderna's pre-clinical studies, clinical trials in human volunteers, manufacturing and quality controls, and assessed that the benefits outweighed the risks for use of the bivalent vaccine as a booster to protect against COVID-19 as the virus continues to evolve.
The HSA will continue to actively monitor the safety of the vaccine and will take necessary actions if significant safety concerns are identified, it noted.
According to data from Singapore's Ministry of Health, as of Wednesday noon, the country recorded some 1.87 million total COVID-19 cases with the death toll at 1,604.
Source: Xinhua